Investigating the NGLY1 / NRF1 Network
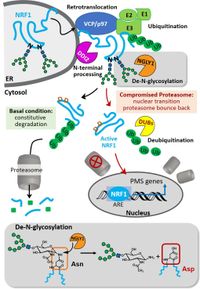
N-Glycanase 1 (NGLY1) is an enzyme that removes N-glycans from glycoproteins that are targeted to ER-associated degradation (ERAD). The heterozygous inactivating mutations in the ngly1 gene causes a rare genetic disorder, called NGLY1 deficiency, which represents a special type of a CDG as in this case a de-glycosylating enzyme is affected by the genetic mutation. Patients exhibit a spectrum of severe symptoms, such as developmental delay, hypotonia, seizures, and peripheral neuropathy. Recently, we were able to show that NGLY1 is essential for activating an important transcription factor Nuclear Factor, Erythroid 2 Like 1 (NFE2L1, also called NRF1), which responds to proteasome insufficiency or pharmacological inhibition by upregulating proteasome subunit gene expression. Chemical or genetic disruption of NGLY1 activity results in the accumulation of mis-processed NRF1 that shows no activation of target genes upon proteasome inhibition. NRF1 is also an attractive but inaccessible target for the therapy of multiple myeloma (MM) that shows a high tendency for resistance mechanisms, involving this transcription factor. Through a small molecule screen, we identified a cell-active NGLY1 inhibitor (WRR139) that disrupts the processing and function of Nrf1. The compound potentiates the cytotoxicity of carfilzomib, a clinically used proteasome inhibitor, against MM and T cell-derived acute lymphoblastic leukemia (T-ALL) cell lines. NGLY1 inhibition prevents NRF1 activation and represents a new therapeutic approach for cancers that depend on proteasome homeostasis.
Current research focuses on the identification of additional NGLY1 substrates and interaction partners. In addition, we are working on new concepts for improving chemical NGLY1 inhibition.