Bioorthogonal Probes for Multimodal Glycoprotein Analysis
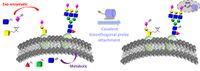
Due to diversity and heterogeneity, the characterization of glycans and glycoproteins is still very challenging, there is a huge need for molecular tools that allow a better characterization of these biomolecules. As a non-templated, posttranslational modification, glycans are not amenable to investigations by traditional genetic techniques. Chemical glycan engineering (metabolic or exo-enzymatic) and subsequent bioorthogonal chemistry can alleviate some of common problems associated with glycoprotein analysis. This concept uses incorporation of chemical handles into glycoproteins that allow the covalent attachment of analytical probes without exhibiting cross reactivity with the natural environment (bioorthogonal).
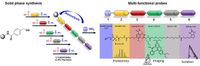
To better analyze glycoproteins in our research, we develop new bioorthogonal probes with a broad application spectrum. By using the principle of solid phase peptide synthesis, we combine multiple functional units (i.e., bioorthogonal handles, fluorophores, isotopic labels, cleavable linkers and affinity tags) in a building block fashion. This fast and easy synthesis strategy offers a free choice of combining a huge variety of analytical read-out options with every available bioorthogonal attachment chemistry. Our probes can be used for qualitative and quantitative analysis of glycoproteins and other biomolecules in multiple applications such as imaging, mass spectrometry, isolation and purification. We will use these probes to characterize aberrant protein glycosylation in certain Congenital Disorders of Glycosylation (CDGs), various types of cancer and to study the infection processes of pathogens.